Drug Nanocarriers for Cancer Chemotherapy

Our new type of nanocarriers is based on a unique modular platform designed to integrate every characteristic of an ideal drug delivery system. This novel platform possesses two main advantages over already existing delivery systems. First, its design supports the creation of an all-in-one delivery system, displaying active targeting and imaging capabilities, as well as releasing a very large payload (up to several thousands of drugs) of several types of therapeutics simultaneously and in a controlled manner. Second, this platform endows the nanocarriers with great versatility, so that they can be easily adapted for use in the treatment of a variety of diseases.
Cancer chemotherapy represents the main area in which these drug nanocarriers are expected to have a tremendous effect. By dramatically reducing side effects and optimizing the therapeutic effect of drugs, they should ideally lead to the potential eradication of many challenging cancers. Consequently, the projects in my group concern multifunctional nanocarriers for cancer chemotherapy, in particular for hormone-refractory prostate cancer.
Specifically, our group has designed a highly stable, dendronized gold nanoparticle (AuNP)-based drug delivery platform, using a novel poly(propyleneimine) (PPI) dendron. The prepared dendronized AuNPs are able to withstand several rounds of lyophilization cycles with no sign of aggregation, are stable in phosphate-buffered saline and Hanks’ buffer as well as in serum, and are resistant to degradation by glutathione exchange reactions. This nanocarrier platform displays a dense coating, with >1400 dendrons/AuNPs, which will enable very high payload.
This platform has been used to design multifunctional AuNPs coated with PPI dendrons that contain both prostate cancer active targeting and chemotherapeutic drugs. The PPI dendron is a good candidate for the design of drug 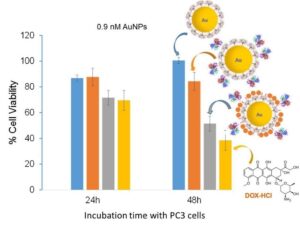 delivery vehicles because of its ability to in-duce a proton-sponge effect that will enhance lysosomal escape and intracellular therapeutic de-livery. On of the chemotherapeutic drug used is doxorubicin (DOX) and it was linked to the dendron through a hydrazine acid-sensitive bond. Subsequent acidification of the AuNP system to pH 4-5 resulted in the release of 140 DOX drugs per nanoparticle. In addition, the PPI-dendron was conjugated via Click chemistry to an EphA2-targeting antibody Fab fragment that has been shown to target prostate cancer cells. In vitro cell viability assays revealed an IC50 of 0.9 nM for the targeted DOX-bearing AuNPs after 48h incubation with PC3 cells. These results are very promising and we are now optimizing the system. Other drugs being investigated are cisplatin and docetaxel.
delivery vehicles because of its ability to in-duce a proton-sponge effect that will enhance lysosomal escape and intracellular therapeutic de-livery. On of the chemotherapeutic drug used is doxorubicin (DOX) and it was linked to the dendron through a hydrazine acid-sensitive bond. Subsequent acidification of the AuNP system to pH 4-5 resulted in the release of 140 DOX drugs per nanoparticle. In addition, the PPI-dendron was conjugated via Click chemistry to an EphA2-targeting antibody Fab fragment that has been shown to target prostate cancer cells. In vitro cell viability assays revealed an IC50 of 0.9 nM for the targeted DOX-bearing AuNPs after 48h incubation with PC3 cells. These results are very promising and we are now optimizing the system. Other drugs being investigated are cisplatin and docetaxel.
In addition, we have recently developed gold nanorattles (AuNRTs) for use in cancer combination therapy (chemotherapy + photothermal therapy). While adapting the Xia’s method (normally used for gold nanocage 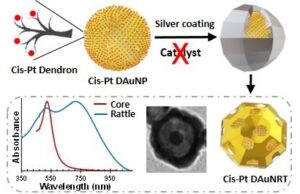 preparation) to the formation of AuNRTs, we discovered that the dendron coating on our AuNPs is sufficient to efficiently catalyze the reduction of silver nitrate during the deposition of the silver shell using a polyol process. Consequently, the very hygroscopic hydrogen sulfide catalyst typically used for this reaction could be omitted, which led to very monodisperse and compact AuNRTs (< 60 nm in diameter). In addition, we demonstrated that the dendron can also be used to load a cargo onto the AuNP core (e.g., around 1,000 cisplatin molecules per AuNP) prior to the nanorattle preparation and still lead to similar nanorattle structures retaining close to 70% of the original cargo. Furthermore, we have observed the controlled release of cisplatin under acidic conditions.
preparation) to the formation of AuNRTs, we discovered that the dendron coating on our AuNPs is sufficient to efficiently catalyze the reduction of silver nitrate during the deposition of the silver shell using a polyol process. Consequently, the very hygroscopic hydrogen sulfide catalyst typically used for this reaction could be omitted, which led to very monodisperse and compact AuNRTs (< 60 nm in diameter). In addition, we demonstrated that the dendron can also be used to load a cargo onto the AuNP core (e.g., around 1,000 cisplatin molecules per AuNP) prior to the nanorattle preparation and still lead to similar nanorattle structures retaining close to 70% of the original cargo. Furthermore, we have observed the controlled release of cisplatin under acidic conditions.
Hyperthermia for Enhanced Delivery of Nanoparticles to Tumors
Advancements in nanotechnology have revolutionized cancer treatment by conjugating therapeutic drugs onto nanocarriers for targeted delivery into tumors while reducing systemic toxicity. However, successful nanostructure 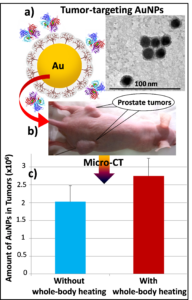 transport from tumor capillaries to tumor interstitial space and diffusion to the entire tumor region are still difficult to achieve, largely due to the large flow resistance caused by small pores in the capillary and high interstitial pressure in tumors. Previous studies have strongly suggested that hyperthermia would be a viable approach to overcome these two barriers. In this funded NSF grand, we have performed in vivo experimental studies to evaluate the effects of whole body and local mild hyperthermia on the deposition of nanoparticles in prostate tumors implanted in mice, and to measure body and tumor temperatures, tumor interstitial pressure, and local blood perfusion rates during experiments. MicroCT imaging was used to scan all the tumors resected after the experiments to determine both the 3-D nanoparticle distribution and the total amount of nanoparticle deposition in tumors. These results suggest mild whole body hyperthermia as a practical approach for overcoming transport barriers of nanoparticle delivery in prostatic prostate tumors.
transport from tumor capillaries to tumor interstitial space and diffusion to the entire tumor region are still difficult to achieve, largely due to the large flow resistance caused by small pores in the capillary and high interstitial pressure in tumors. Previous studies have strongly suggested that hyperthermia would be a viable approach to overcome these two barriers. In this funded NSF grand, we have performed in vivo experimental studies to evaluate the effects of whole body and local mild hyperthermia on the deposition of nanoparticles in prostate tumors implanted in mice, and to measure body and tumor temperatures, tumor interstitial pressure, and local blood perfusion rates during experiments. MicroCT imaging was used to scan all the tumors resected after the experiments to determine both the 3-D nanoparticle distribution and the total amount of nanoparticle deposition in tumors. These results suggest mild whole body hyperthermia as a practical approach for overcoming transport barriers of nanoparticle delivery in prostatic prostate tumors.
Nanoparticle-mediated High-Intensity Focused Ultrasound (HIFU) for Enhanced Thermal Ablation of Tumors
Nanoparticles (NPs)-mediated high intensity focused ultrasound (HIFU) is a minimally invasive surgical technique with significant potential for improving thermal ablation of cancers, uterine fibroids, and other malignant tissues. The Banerjee lab’s recent work has shown that HIFU heat deposition within the targeted tumor area and irreversible tumor cell damage are considerably enhanced by directly injected AuNPs. This approach reduces the need for high levels of HIFU power and minimizes thermal damage in adjacent normal tissues.
HIFU experiments with Fab-functionalized AuNPs (Fab-AuNPs) were conducted, and results were analyzed. The  presence of Fab-AuNPs led to increase of the temperature rise compared to baseline. Furthermore, increase in concentration of Fab-AuNPs resulted in increased temperature rise. HIFU treatment at 50W power level and Fab-AuNPs concentration of 0.125% showed maximum temperature rise while producing structural tumor tissue damage. The presence of Fab-AuNPs produced higher thermal dose compared to baseline. Additionally, increased value of thermal dose was observed for increased concentration of Fab-AuNPs. Furthermore, sonication time was reduced significantly with the use of Fab-AuNPs. These findings indicate that the enhancement of local energy delivery during HIFU sonication can be achieved with the use of Fab-functionalized AuNPs.
presence of Fab-AuNPs led to increase of the temperature rise compared to baseline. Furthermore, increase in concentration of Fab-AuNPs resulted in increased temperature rise. HIFU treatment at 50W power level and Fab-AuNPs concentration of 0.125% showed maximum temperature rise while producing structural tumor tissue damage. The presence of Fab-AuNPs produced higher thermal dose compared to baseline. Additionally, increased value of thermal dose was observed for increased concentration of Fab-AuNPs. Furthermore, sonication time was reduced significantly with the use of Fab-AuNPs. These findings indicate that the enhancement of local energy delivery during HIFU sonication can be achieved with the use of Fab-functionalized AuNPs.
Our long-term goal is to contribute toward the development of highly effective NPs-mediated HIFU ablation procedures and expand their range to include targeted imaging and treatment of both deep-seated tumors and metastatic sites. The overall objective is to achieve focused ablation at reduced HIFU power levels using functionalized NPs-enhanced energy deposition. The central hypothesis is that HIFU ablation using systemically introduced functionalized NPs will increase precision targeting of localized tumors and enable uniform heat deposition in the target area, allowing for large volumes of cell necrosis at relatively low powers. Furthermore, it could facilitate targeting of metastasizing PCa cells and/or lesions.
Controlled Assembly of Inorganic Nanoparticles for the Formation of new Hybrid Nanomaterials
The goal of this collaborative project between the Pelton (Physics, UMBC) and Daniel research groups is to couple metal nanoparticles to semiconductor nanoparticles to modify the properties of the individual component particles. The coupling of nanoparticles has been used to enhance scattering, fluorescence, and absorption, leading to 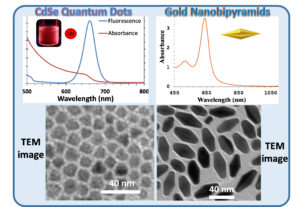 applications in sensing, photocatalysis, and light harvesting. However, the exact properties that emerge are highly dependent on the precise arrangement and number of the coupled nanoparticles, and creating uniform, discrete assemblies remains a challenge. In particular, the coupling of plasmonic and excitonic nanoparticles has untapped potential if strong coupling between their respective plasmons and excitons can be reliably achieved. Novel properties, for example Fano interference and Rabi splitting, are expected to arise when these particles are strongly coupled. But coupling strength is especially dependent on the precise position of the component particles. Nevertheless, a system composed of a single emitter quantum dot (QD) strongly coupled to plasmonic nanoparticles would have widespread applications in photonics, optics, and information processing, making such a system desirable.
applications in sensing, photocatalysis, and light harvesting. However, the exact properties that emerge are highly dependent on the precise arrangement and number of the coupled nanoparticles, and creating uniform, discrete assemblies remains a challenge. In particular, the coupling of plasmonic and excitonic nanoparticles has untapped potential if strong coupling between their respective plasmons and excitons can be reliably achieved. Novel properties, for example Fano interference and Rabi splitting, are expected to arise when these particles are strongly coupled. But coupling strength is especially dependent on the precise position of the component particles. Nevertheless, a system composed of a single emitter quantum dot (QD) strongly coupled to plasmonic nanoparticles would have widespread applications in photonics, optics, and information processing, making such a system desirable.
To this end, Pelton and Daniel are investigating the assembly and optical properties of gold nanoparticles-CdSe/CdS QDs conjugates. The Daniel’s lab has linked excitonic nanoparticles (CdSe/CdS QDs) to AuNPs through covalent bonds. Strong plasmon-exciton coupling was achieved between a single CdSe/CdS QD and the gap plasmon formed between a gold nanosphere and a silver film, providing the first definitive evidence of strong coupling between a single emitter and a gap plasmon. This study revealed that the yield of strongly coupled particles was limited by the geometry of nanospheres. To address this, we created hybrid nanostructures composed of end-to-end pairs of gold bipyramids (AuBPs) with a single CdSe/CdS QD between the two AuBPs. The assembly was achieved through copper-free click chemistry bet the design and assembly of hybrid nanostructures for plasmon-exciton coupling, composed of end-to-end pairs of gold bipyramids (AuBPs) with a single CdSe/CdS quantum dot (QD) between the two AuBPs. 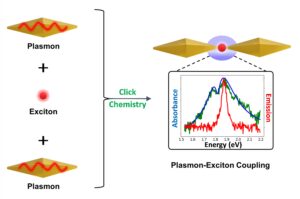 The assembly was achieved through copper-free click chemistry between azide-functionalized AuBPs and dibenzocyclooctyne (DBCO)-modified QDs, providing efficient and strong linkage between nanoparticles. Discrete AuBP-QD assemblies (with 1 or 2 AuBPs) accounted for 47% of the resulting nanoconstructs. The functionalization and assembly of the nanoparticles was verified through infrared and visible absorption spectroscopy, fluorescence spectroscopy, zeta potential measurements, and transmission electron microscopy. Measurements on single assemblies showed an induced transparency in the plasmon scattering spectrum, characteristic of intermediate coupling with excitons in the QD. A coupling strength of 45 meV was achieved for single QDs at room temperature. These results highlight the potential of colloidal AuBP-QD assemblies for achieving strong plasmon-exciton coupling using a directed assembly approach enabled by an efficient click chemistry strategy. ween azide-functionalized AuBPs and dibenzocyclooctyne (DBCO)-modified QDs, providing efficient and strong linkage between nanoparticles. Discrete AuBP-QD assemblies (with 1 or 2 AuBPs) accounted for 47% of the resulting nanoconstructs. The functionalization and assembly of the nanoparticles was verified through infrared and visible absorption spectroscopy, fluorescence spectroscopy, zeta potential measurements, and transmission electron microscopy. Measurements on single assemblies showed an induced transparency in the plasmon scattering spectrum, characteristic of intermediate coupling with excitons in the QD. A coupling strength of 45 meV was achieved for single QDs at room temperature. These results highlight the potential of colloidal AuBP-QD assemblies for achieving strong plasmon-exciton coupling using a directed assembly approach enabled by an efficient click chemistry strategy.
The assembly was achieved through copper-free click chemistry between azide-functionalized AuBPs and dibenzocyclooctyne (DBCO)-modified QDs, providing efficient and strong linkage between nanoparticles. Discrete AuBP-QD assemblies (with 1 or 2 AuBPs) accounted for 47% of the resulting nanoconstructs. The functionalization and assembly of the nanoparticles was verified through infrared and visible absorption spectroscopy, fluorescence spectroscopy, zeta potential measurements, and transmission electron microscopy. Measurements on single assemblies showed an induced transparency in the plasmon scattering spectrum, characteristic of intermediate coupling with excitons in the QD. A coupling strength of 45 meV was achieved for single QDs at room temperature. These results highlight the potential of colloidal AuBP-QD assemblies for achieving strong plasmon-exciton coupling using a directed assembly approach enabled by an efficient click chemistry strategy. ween azide-functionalized AuBPs and dibenzocyclooctyne (DBCO)-modified QDs, providing efficient and strong linkage between nanoparticles. Discrete AuBP-QD assemblies (with 1 or 2 AuBPs) accounted for 47% of the resulting nanoconstructs. The functionalization and assembly of the nanoparticles was verified through infrared and visible absorption spectroscopy, fluorescence spectroscopy, zeta potential measurements, and transmission electron microscopy. Measurements on single assemblies showed an induced transparency in the plasmon scattering spectrum, characteristic of intermediate coupling with excitons in the QD. A coupling strength of 45 meV was achieved for single QDs at room temperature. These results highlight the potential of colloidal AuBP-QD assemblies for achieving strong plasmon-exciton coupling using a directed assembly approach enabled by an efficient click chemistry strategy.
Gold Nanoparticle-based Microfluidic Device for Lead Sensing in Tap Water
Lead contamination in tap water remains a significant public health concern in the United States, with low exposure levels causing severe health issues, especially in children. This project aims to develop a cheap and user-friendly microfluidic device that utilizes an AuNP-based colorimetric assay to detect lead in water. In phase one of the project,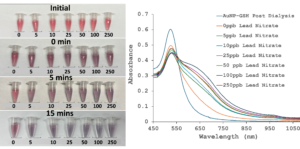 20 nm citrate-coated AuNPs were synthesized with a polydispersity index (PDI) of less than 0.2, ensuring uniformity, and functionalized with L-glutathione. A lead detection assay was then performed by adding water samples with lead concentrations ranging from 0 to 250 ppb to functionalized AuNPs spiked with NaCl. Results showed that samples containing 5 ppb or more of lead, induced nanoparticle aggregations, shifting the solution color from red to blue. In contrast, the zero ppb control sample remained red. These preliminary results demonstrate the assay’s effectiveness in detecting lead at the FDA’s toxicity threshold of five ppb. Current efforts focus on improving reproducibility and integrating the assay into a portable microfluidic device for rapid home testing. By providing a cost-effective and accessible lead detection method, this research contributes to public health by enabling individuals to monitor the safety of their drinking water.
20 nm citrate-coated AuNPs were synthesized with a polydispersity index (PDI) of less than 0.2, ensuring uniformity, and functionalized with L-glutathione. A lead detection assay was then performed by adding water samples with lead concentrations ranging from 0 to 250 ppb to functionalized AuNPs spiked with NaCl. Results showed that samples containing 5 ppb or more of lead, induced nanoparticle aggregations, shifting the solution color from red to blue. In contrast, the zero ppb control sample remained red. These preliminary results demonstrate the assay’s effectiveness in detecting lead at the FDA’s toxicity threshold of five ppb. Current efforts focus on improving reproducibility and integrating the assay into a portable microfluidic device for rapid home testing. By providing a cost-effective and accessible lead detection method, this research contributes to public health by enabling individuals to monitor the safety of their drinking water.